GSSG Application
Revision 05/17/12
Overview
Reduced glutathione (GSH) plays a primary role in the protection of the cell against oxidative damage. Two important antioxidant functions include the reduction of hydrogen peroxide (H2O2) to water and scavenging free radicals such as the hydroxyl radical (HO•). An increase in H2O2, the presence of other reactive species, or interference with the GSH redox cycle can result in an increase in oxidized glutathione (GSSG).
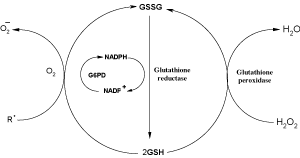 Figure 1.
Figure 1.
The NWLSS™GSH01 Assay standard method measures "total" glutathione (GSHt). The GSHt in the sample is defined as the concentrations of GSH and GSSG (The mixed disulfides of GSH and cysteine or proteins are not included in the definition). However, it is also possible to measure the GSSG concentration of the sample by first treating the sample with 4-vinylpyridine (4-VP) to sequester GSH.
The estimated GSSG concentration is highly influenced by sample history and treatment. Ex vivo, GSSG concentration can increase due to continued reduction of hydrogen peroxide by glutathione peroxidase and reaction with other reactive species. This is especially true for cells that have been lysed, even when refrigerated. Conversely, the GSSG concentration in the sample can be under estimated if the glutathione redox cycle is active or by mixed disulfide reactions with sulfhydryls other than GSH. These issues can be minimized by maintaining the sample at 0°C (ice bath) and extracting with metaphosphoric acid as soon as possible. Once extracted, the protein free acidic sample can be stored at -20°C for several months or indefinitely at -80°C.
GSH Scavenging
4-Vinylpyridine was added to a buffer sample containing 1 mM GSH at 18°C (final concentration 4VP is approximately 10 mM). At various time intervals, the GSH was measured by reaction with an excess of 5-5'-dithiobis-2-nitrobenzoic acid (DTNB). The percent remaining GSH was then plotted as a function of time. As shown in Figure 2, 100% of the GSH is removed within 60 minutes.
Calibration
The presence of 4-VP and the reduction in sample dilution requires that the calibrators be treated the same as the test samples. The resulting calibration curve will show significantly reduced reaction rates. As seen in Figure 4 below, the calibration curve maintains linearity.
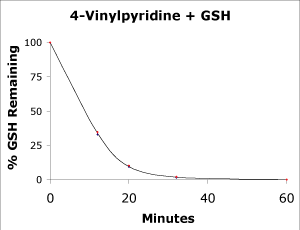 Figure 2.
Figure 2.
Procedure
The procedure for the GSSG application using the NWK-GSH01 assay is essentially the same as the standard method, with the following exceptions:
- The calibrators are made up using an MPA:Assay Buffer solution (2:1) instead of Assay Buffer.
- Calibrators are treated with 4VP reagent the same as samples
- 4 N Sodium hydroxide (NaOH) is required but not provided.
- Reagent grade methanol or ethanol is required but not provided
- 4-Vinylpyridine is required but not provided.
- Extended reaction time
Reagent Preparation
Prepare 4 N NaOH
Add 15 mL water to flask
Add 5.2 mL 50% NaOH (19.4 N) to 25 ml volumetric flask
Mix
Cool to room temperature
Bring volume to 25 mL with water
Store in a plastic bottle at room temperature
Add 4 g NaOH pellets to 25 ml volumetric flask
Add 20 mL water volumetric flask
Mix until dissolved
Cool to room temperature
Bring volume to 25 mL with water
Store in a plastic bottle at room temperature
Prepare calibrators in a 2 to 1 MPA:Assay Buffer Solution
0 uM GSSG - MPA:Assay Buffer
5 uM GSSG - 25 uL calibrator concentrate in 975 uL MPA:Assay Buffer Solution
10 uM GSSG - 50 uL calibrator concentrate in 950 uL MPA:Assay Buffer Solution
Note: Calibrators will be treated and assayed the same as the samples except they don't need to be frozen.
Prepare 4-Vinylpyridine
4-vinylpyridine is not supplied with the NWK-GSH01 assay but is available commercially (Aldrich #V320-4 or equivalent, CAS 100-43-6). The amount of 4-VP required per assay is small; therefore it is recommended that one purchase the minimum quantity of reagent.
- 4-VP is typically stored at -20°C and must be brought to ambient temperature before opening the container.
- Add 1 mL of methanol or ethanol to a glass amber vial or other suitable vial with closure.
- Add 60 µL 4-VP to produce an approximate 500 mM 4VP solution.
- Close vial and store on ice.
- The diluted 4-VP should be used on the same day of prepararation.
Test 4-VP solution (optional)
4-Vinylpyridine darkens with age without affecting the purity (Sigma-Aldrich Specification Sheet). The activity of 4-VP can be verified by taking advantage of the reaction of DTNB with GSH. Note: The ready-to-use DTNB Reagent is supplied with sufficient volume to perform one 4-VP verification.
- Prepare a 10 mM solution of GSH in Assay Buffer or equivalent buffer at pH 7-8
- Stopper tubes and incubate at room temperature for 1 hour.
- Add 100 µL DTNB Reagent to all tubes.
- Incubate for 15-30 minutes.
- Measure the absorbance at 412 nm
- Calculate the %GSH remaining. (Test-Negative Control)/(Positive Control - Negative Control)
| Sample | GSH | Buffer | 4-VP |
|---|---|---|---|
| Positive Control, µL | 100 | 800 | 0 |
| Test, µL | 100 | 800 | 10 |
| Negative Control, µL | 0 | 900 | 10 |
5% MPA
- Prepare a 5% w/v solution of MPA, place on ice.
Sample Processing
Below is a recommended sample processing procedure for tissue samples. Other sample types may require a modified procedure. In the case of whole blood, the sample would be directly extracted in 5% MPA. Note that the assay requires 200 µL clarified homogenate supernatant that has been extracted with MPA solution (2:1 MPA/Assay Buffer) to measure both GSH and GSSG.
- Samples must be immediately placed at 0°C. If the samples cannot be processed immediately, store at -80°
- Prepare an ice cold 10-20% wet weight/volume homogenate; e.g. 100 mg in 500 µL buffer.
- Transfer the sample homogenate to a microcentrifuge tube.
- Centrifuge at 10,000 xg for 2 minutes (or sufficient to produce a clear supernatant)
- Collect the supernatant and aliquot an appropriate portion for protein analysis.
Extract Clarified Sample Homogenates in MPA to Precipitate Proteins Prior to Assay
- Add 150 µL clarified sample homogenate (supernatant) to microcentrifuge tube.
- Add 150 µL 5% MPA to microcentrifuge tube.
- Vortex (5 count).
- Centrifuge at 10,000 xg for 2 minutes (or sufficiently to pellet the precipitate)
- Place the extract on ice or store at -20°C until assay. (Use undiluted extract for GSSG testing below or dilute extract 1/20 before testing for "total GSH" according to standard procedure, page 8 of NWK-GSH01 product insert.
pH Adjustment and Treament with 4VP Scavenger Reagent
- Add 150 µL undiluted Sample Extract or Calibrator to a microcentrifuge tube.
- Add 7.5 µL 4 N NaOH, mix. (This is necessary to bring the Sample Extract and Calibrators to near neutral pH before addition of 4-VP scavenger reagent
- Add 10 µL diluted 4-VP (525 mM), mix. (4VP will be 9.7 mM in final reaction mix
- Add 350 µL Assay Buffer, mix.
- Incubate at room temperature for 1 hour.
- Proceed with the assay protocol below without additional dilution of sample extracts or calibrators.
Assay
- Remove microplate from plastic bag.
- Bring all reagents to room temperature.
- Add 50 µL of calibrator or sample extract to a designated well.
- Add 50 µL of DTNB to well.
- Add 50 µL of GR to well.
- Incubate microplate for 3-5 minutes at room temperature.
- Add 50 µL of reconstituted NADPH to wells containing calibrator, control or sample.
- Begin recording the absorbance at 405 nm (412 if possible) at 15-20 second intervals for 5 minutes.
- Determine the concentration of the controls and samples. If using data reduction on the plate reader, skip steps a-c.Important Note:
- Calculate the rate for each calibrator, control and sample from the linear regression of A405 as a function of time.
- Calculate the linear regression parameters to obtain the equation of the line.
- Calculate the concentrations of the controls and samples.
- Correct the control and samples for dilution and report results.




